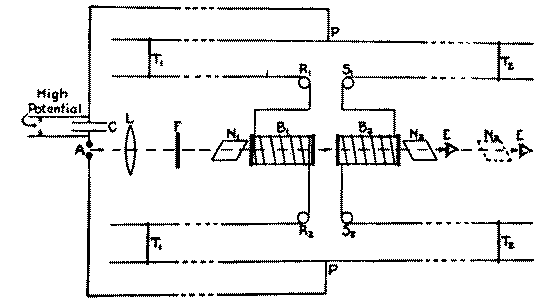
L'effet �tait tr�s simple. Il y a l'effet Faraday par lequel un faisceau de lumi�re polaris�e passant � travers un liquide dans lequel un champ magn�tique est en rotation -- le plan de polarisation est tourn� par un champ magn�tique longitudinal. Maintenant cette id�e a �t� mieux connue pendant un long moment et elle eut beaucoup d'importance par rapport aux volets de lumi�re. At any rate, you can let light through or not depending upon the magnetic field. Now the experiment of Allison's was this (Fig. 3). They had a glass cell and a coil of wire around it (B1, B2) and you have wires coming up here, a Lecher system. Here you have a spark gap, so a flash of light comes through here and goes through a Nicol prism over here and another one over here, and you adjust this one with a liquid like water or carbon disulfide or something like that in the cell so that there was a steady light over here. If you have a beam of light and you polarize it and then you turn on a magnetic field, why you see that you could rotate the plane of polarization. There will be an increase in the brightness of the light when you put a magnetic field on here. Now they wanted to find the time delay, how long it takes. So they had a spark and the same field that produced the spark induced a current through the coil, and by sliding this wire along the trolley of the Lecher system, they could cause a compensating delay. The sensivity of this thing was so great that they could detect differences of about 3 x 10-10 seconds. By looking in here they could see these flashes of light, the light from the sparks, and they tried to decide as they changed the position of this trolley whether it got brighter or dimmer and they set it for a minimum, and measured the position of the trolley. They put in here--in this glass tube--they put a water solution and added some salt to it. And they found that the time lag was changed, so that they got a change in the time lag depending upon the presence of salts.
Now they first found--very quickly--that if you put in a thing like ethyl alcohol that you got one characteristic time lag, and with acetic acid another one, quite different. But if you had ethyl acetate you got the sum of the two. You got two peaks. So that you could analyze ethyl acetate and find the acetic acid and the ethyl alcohol. Then they began to study salt solutions and they found that only the metal elements counted but they didn't act as an ion. That is, all potassium ions weren't the same, but potassium nitrate and potassium chloride and potassium sulfate all had quite characteristic different points, that were a characteristic of the compound. It was only the positive ion that counted and yet the negative ions had a modifying effect. But you couldn't detect the negative ions directly.
Now they began to see how sensitive it was. Well, they found that any intensity more than about 10-8 molar solution would always produce the maximum effect, and you'd think that that would be kind of discouraging from the analytical point of view, but no, not at all. And you could make quantitative measurements to about three significant figures by diluting the solutions down to a point where the effect disappeared. Apparently, it disappeared quite sharply when you got down to about 10-8 or 3. 42 x 10-8 in concentration, or something of that sort and then the effect would disappear. Otherwise, you would get it, so that you could detect the limit within this extraordinary degree of accuracy.
Well, they found that things were entirely different, even in these very dilute solutions, in sodium nitrate from what it was with sodium chloride. Nevertheless, it was a characteristic which depended upon the compound even though the compound was disassociated into ions at those concentrations. That didn't make any difference but it was fact that was experimentally proven. They then went on to find that the isotopes all stick right out like sore thumbs with great regularity. In the case of lead, they found sixteen isotopes. These isotopes were quite regularly spaced so that you could get 16 different positions and you could assign numbers to those so that you can identify them and tell which they are. Unfortunately, you couldn't get the concentrations quantitatively, even the dilution method didn't work quite right because they weren't all equally sensitive. You could get them relatively but only approximately. Well, it became important as a means of detecting elements that hadn't yet been discovered, like Alabamine and elements that are now known, and filling out the periodic table. (p.8) All the elements in the periodic table were filled out that way and published.
But a little later, in 1945 or 46, I was at the University of California. Owen Latimer who is now Head of the Chemistry Department there--not Owen Latimer, Wendell Latimer--had had a bet with G. N. Lewis (in 1932). He said, "There's something funny about this Allison effect, how they can detect isotopes." He had known somebody who had been down with Allison and who had been very much impressed by the effect and he said to Lewis, "I think I'll go down and see Allison, to Alabama, and see what there is in it. I'd like to use some of these methods."
Now people had begun to talk about spectroscopic evidence that there might be traces of hydrogen of atomic weight three. It wasn't spoken of as tritium at that time but hydrogen of atomic weight three that might exist in small amounts. There was a little spectroscopic evidence for it and Latimer said, "Well, this might be a way of finding it. I'd like to be able to find it." So he went and spent three weeks at Alabama with Allison and before he went he talked it over with G. N. Lewis about what he thought the prospects were and Lewis said, "I'll bet you ten dollars you'll find that there's nothing in it." And so they had this bet on. He went down there and he came back. He set up the apparatus and made it work so well that G. N. Lewis paid him the ten dollars. (Laughter) He then discovered tritium and he published an article in the Physical Review.(10) Just a little short note saying that using Allison's method he had detected the isotope of hydrogen of atomic weight three. And he made some sort of estimate as to its concentration.
Well, nothing more was heard about it. I saw him then, seven or eight years after that. I had written these things up before, about this Allison effect, and I told him about this point of view and how the Allison effect fits all these characteristics. Well, I know at that time at one of the meetings of the American Chemical Society there was great discussion as to whether to accept papers on the Allison effect. There they decided: No, they would not accept any more papers on the Allison effect, and I guess the Physical Review did too. At any rate, the American Chemical Society decided that they would not accept any more manuscripts on the Allison effect. However, after they had adopted that as a firm policy, they did accept one more a year or two later because here was a case where all the people in the faculty here had chosen twenty or thirty different solutions that they had made up and they had labeled them all secretly and they had taken every precaution to make sure that nobody knew what was in these solutions, and they had given them to Allison and he had used his method on them and he had gotten them all right, although many of them were at concentrations of 10-6 and so on, molar. That was sufficiently definite -- good experimental methods -- and it was accepted for publication by the American Chemical Society but that was the last.(11) You'd think that would be the beginning, not the end.
Anyway, Latimer said, "You know, I don't know what was wrong with me at that time,' He said, "After I published that paper I never could repeat the experiments again. I haven't the least idea why." "But," he said, "Those results were wonderful, I showed them to G. N. Lewis and we both agreed that it was all right. They were clean cut. I checked myself every way I knew how to. I don't know what else I could have done, but later on I just couldn't ever do it again."
I don't know what it is. That's the kind of thing that happens in all of these. All the people who had anything to do with these things find that when you get through with them--you can't account for Bergen Davis saying that they didn't calculate those things from the Bohr theory, that they were found by empirical methods without any idea of the theory. Barnes made the experiments, brought them in to Davis, and Davis calculated them up and discovered all of a sudden that they fit the Bohr theory. He said Barnes didn't have anything to do with that. Well, take it or leave it, how did he do it? It's up to you to decide. I can't account for it. All I know is that there was nothing salvaged at the end, and therefore none of it was ever right, and Barnes never did see a peak. You can't have a thing halfway right.
| Home > Science pathologique |
|---|